Certificate of CE
Jun 12,2020
- Period:
- Apr 15,2019 - Apr 14,2025
- Certification bodies:
- NQA
This is to hereby declare that the devices of this declaration comply with European Council Directive 93/42/EEC and its amendments concerning medical devices.
EN ISO 14971:2012
EN ISO15223-1:2016
EN 1041:2008+A1:2013
EN 60601-1:2006/A1:2013
EN 60601-1-2:2015
EN 12184:2014
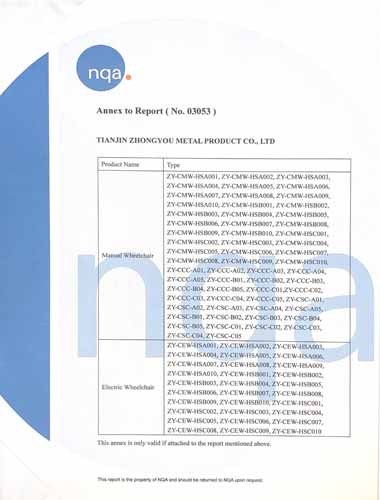
The certificate is valid for the following scope:Manual Wheelchair, Electric Wheelchair, Rollator, Walking Rollator


subscription
Company
Mobile Web


